Introduction: Growth retardation (GR) and pubertal disturbances are the earliest consequences of iron toxicity resulting from the pituitary iron deposition in children and adolescents with thalassemia major (TM). It is suggested that adequate transfusion regimens together with appropriate management of iron chelation with timely initiation and dose tailoring by close monitoring using surrogate markers of the iron load may achieve a normal pattern of complication-free survival in TM.
Objectives: The purpose of this study was to evaluate the fact that the current approach to the management of the disease has provided a normal growth progression and sexual development in patients (pts) with TM.
Methods: We recruited male (M) and female (F) TM pts born after 2000 and followed-up (FU) in Ege University Thalassemia Center. The pts. with an acute or chronic illness that may interfere with growth and development or stem cell transplantation recipients were excluded. The Committee of Clinical Investigation at Ege University Hospital (19.02.2020, 20-2.1T/2) approved this study.
Height (H) and weight (W) assessments were performed by using the Harpenden stadiometer and puberty was evaluated by Tanner staging at 3 monthly intervals. H-standard deviation scores (h-SDS) were calculated. Target-H based on mid-parental-H for M & F pts was estimated. All pts. were evaluated annually for bone mineral density (BMD) using dual-energy X-ray absorptiometry (Hologic QDR‐4500W, USA) after 8 years (y) of age. The mean SDS of the lumbar spine (L1-4) BMD between -1 and -2.5 was defined as osteopenia and <-2.5 as osteoporosis. Growth hormone (GH) secretion was evaluated by L-dopa and insulin tests in pts. with standing-H below the -2 SD. Pts whose GH response to both stimulation tests were less than 5 ng/ml with the monoclonal assay were considered as GH deficient.
Transfused pRBC units (U) and average pre-transfusion hemoglobin (Hb) per year were recorded. Serum ferritin (SF) was monitored at monthly intervals. Myocardial (T2*) and liver (R2) iron were assessed by a 1.5 Tesla magnetic resonance imaging (MRI) machine (Siemens, Symphony Vision 16034 scanner) after 8 y. Annual median SF, prescribed chelator(s), mean chelator doses were recorded by reviewing pts' records. Transfusional iron intake (TII) was estimated as the total amount of pure RBCs (each U, 250 ml with 57% Hct) transfused in a y (ml) x 1.08. Daily TII was expressed as annual TII (mg)/ mean kg b.w./days of the y.
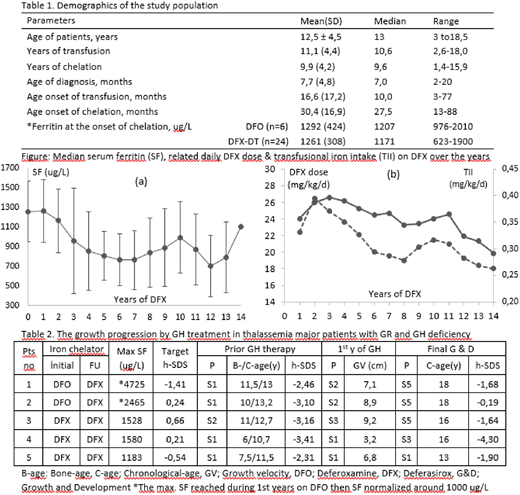
Results: We recruited 30 TM (28 β/β, 1 β/δβ, and 1 non-deletional HbH) pts (13 F and 17 M). Demographics for the population are summarized in Table1. All pts maintained annual mean pre-transfusion Hb> 9 g/dl. Twenty-two of 24 pts maintained Deferasirox (DFX) chelation during 9 ± 3.6 y (median 9, range 2-13 y) and achieved optimum SF levels at all times (Figure a). The dose tailoring of DFX found parallel to the changes in TII during FU (Figure b). Liver iron concentration (LIC) and myocardial T2* (mT2*) in 22 pts on DFX chelation were between 0.9-5.4 (2.1 ± 1.3 SD) mg Fe/g d.w. and 19.4-43.5 (28 ± 6.7 SD) ms, respectively, in all assessments. The only pt with mT2* of 19.4 ms revealed mT2* of >20 ms on FU scans.
The puberty initiated at 12.8 ± 1.1 y in M (n=12) & 11.2 ± 1.3 y in F (n=12) and progressed without hormone replacement in all, regardless of the chelator. Overall, growth reduced compared to the non-thalassemic population after 8 y with clear evidence of growth catch-up by the introduction of puberty. h-SDS detected <-2 in 6 pts in whom one had a familial GR and 5 found GH deficient and received GH therapy. All pts but one developed h-SDS< -2 at 10-12 y-old when they had S1/2 puberty. The pt no 4 who was entirely unresponsive to GH stimulation tests, developed GR at 1 y-old. He remained unresponsive to GH therapy (Table 2). The 14 (%58) and 5 (21%) of 24 pts demonstrated osteopenia and osteoporosis, respectively. Improvement in BMD-SDS was observed following pubertal spurt.
Conclusions: Puberty was the main determinant of growth and bone maturation in TM. Despite, puberty has achieved within physiological limits in M & F pts, a slight delay was observed compared to the non-thalassemic population. The relative delay in pubertal spurt may lead to overdiagnosis of growth and bone-maturation failure and unnecessary intervention in adolescent TM pts. This hypothesis as well as the appropriateness of current management guidelines remains to be elucidated.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal